Atoms and Elements
Introduction
An atom is the smallest particle of an element that retains the chemical identity of that element. An element is composed of only one type of atom. If two or more different atoms form bonds, then the resulting product is called a compound. Hydrogen, the first element in the periodic table, is a diatomic element. This is because in nature, hydrogen gas consists of two hydrogen atoms bonded together. Its chemical formula is ${H_2}$.
Other common diatomic molecules include iodine (${I_2}$), bromine (${Br_2}$), chlorine (${Cl_2}$), fluorine (${F_2}$), oxygen (${O_2}$), and nitrogen (${N_2}$). (These elements can be remembered by using the mnemonic: I Bring Clay For Our New Home).
The law of conservation of mass (also known as the law of conservation of matter) states that after a chemical reaction has taken place, the amount of substances or material present before the reaction is the same as the amount after the reaction. By "amount", I mean the total mass.
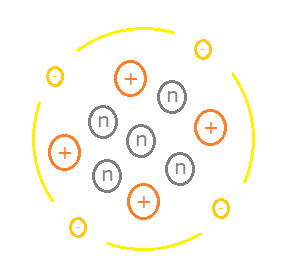
Atoms are composed of subatomic particles, some of which are positively charged (+), some negatively charged (-), and some neutral (no charge). Charged particles with the same type of charge repel one another, while those of opposite types of charge attract one another. We say that "like repels like", and "opposites attract".
The negatively charged particles are called electrons. The positively charged particles are called protons, and the neutral particles are called neutrons. The electronic charge of an electron is ${-1.6} \times 10^{-19}$C, where C is for coulombs, the unit of measurement for electric charge. A proton has a positive charge of the same magnitude but opposite sign, i.e. ${+1.6} \times 10^{-19}$C.
In the simplistic and historical view of the atom, the nucleus of the atom is where the protons and neutrons can be found. Collectively, they are sometimes referred to as nucleons. Electrons orbit around the nucleus (this model is reminiscent of the solar system, where planets orbit the sun due to the force of gravity). This is a convenient way to picture the internal structure of the atom, but it is not quite accurate.
Atoms are incredibly small. In general, the diameter of an atom is within the range of 100 to 500 picometres, which is $100 \times 10^{-12}$m to $500 \times 10^{-12}$ m. It is more convenient to use the non-SI unit known as the angstrom ($\mathring{A}$), which is $1 \times 10^{-10}$ m. This means that an atom's diameter generally lies between 1 to 5 angstroms. Due to the incredibly small masses of atoms, we'll use the atomic mass unit (amu or u), which is $1.661 \times 10^{-27}$ kg.
- Mass of proton: $m_p = 1.0073$ u
- Mass of neutron: $m_n = 1.0087$ u
- Mass of electron: $m_e = 0.0005486$ u
The number of protons inside an atomic nucleus is called the atomic number (Z). The mass number (A) of an element is the number of nucleons (protons plus neutrons). The number of electrons needs to equal the number of protons in order for the atom to be electrically neutral. If an atom is not electrically neutral (e.g. more protons than electrons), then it is called an ion. An ion is an electrically charged atom or molecule.
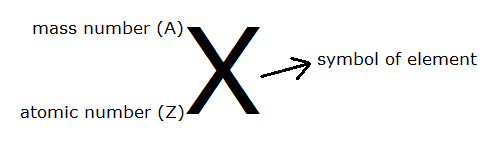
Two atoms with the same number of protons, but a different number of neutrons, are referred to as isotopes of each other. An example of a pair of isotopes is carbon-12 and carbon-14: both have 6 protons, but the former has 6 neutrons and the latter has 8 neutrons. Another example is uranium, which has three isotopes, namely uranium-234, uranium-235, and uranium-238. The number of nucleons inside an atom influences the nuclear properties of the atom.
References
- Theodore L. Brown, H. Eugene LeMay, Jr., Bruce E. Bursten, Catherine J. Murphy, Patrick Woodward, Chemistry: The Central Science - 11th Edition, Pearson Education International, 2009